
What are boyles law? Definition, Types and Importance physics AESL
Boyle's law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure ( p) of a given quantity of gas varies inversely with its volume ( v) at constant temperature; i.e., in equation form, pv = k, a constant.
Boyle’s Law Chemistry Skills
Suppose the gas with pressure P 1 and volume V 1 expands or shrinks to pressure P 2 and volume V 2. Then, using Boyle's law equation, P 1 V 1 = k and P 2 V 2 = k. From the above two equations. P 1 V 1 = P 2 V 2. This equation shows that as the pressure increases, the volume decreases and vice versa. For example, when the pressure doubles, the.

8 Boyle’s Law Examples in Real Life StudiousGuy
Figure 14.3.1 14.3. 1: Robert Boyle. (CC BY-NC; CK-12) Mathematically, Boyle's law can be expressed by the equation: P × V = k P × V = k. The k k is a constant for a given sample of gas and depends only on the mass of the gas and the temperature. The table below shows pressure and volume data for a set amount of gas at a constant temperature.

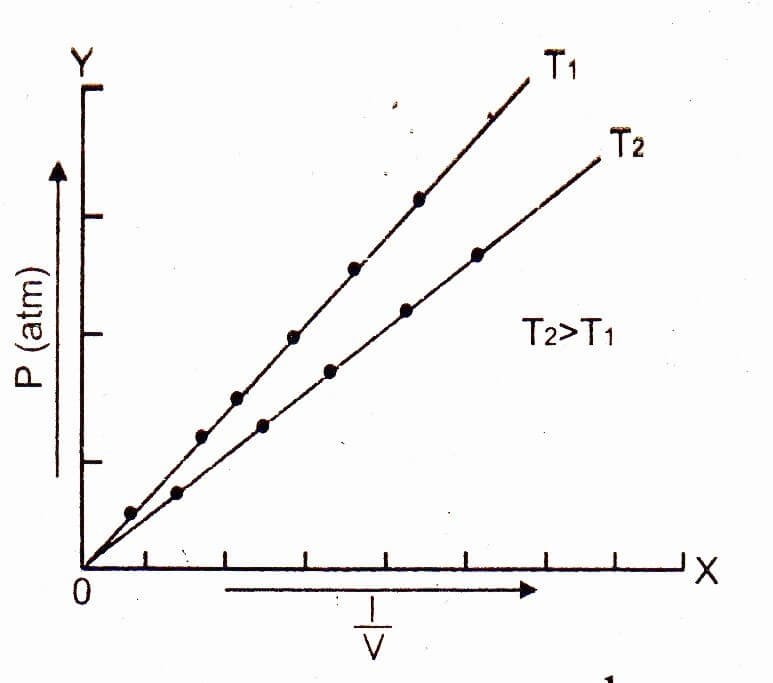
The graph for Boyle's law is called
Robert Boyle's observations are summed up in Boyle's law, which states that for a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure. Because of the inverse relationship, the product of the two quantities, pressure and volume, is constant. When given any two sets of pressure and volume, at a given.

FileBoyles Law.svg Wikipedia, the free encyclopedia
This Boyle's law calculator works in any direction you like. Just insert any three parameters, and the fourth one will be calculated immediately! We can visualize the whole process on Boyle's law graph. The most commonly used type is where the pressure is a volume function. For this process, the curve is a hyperbola.
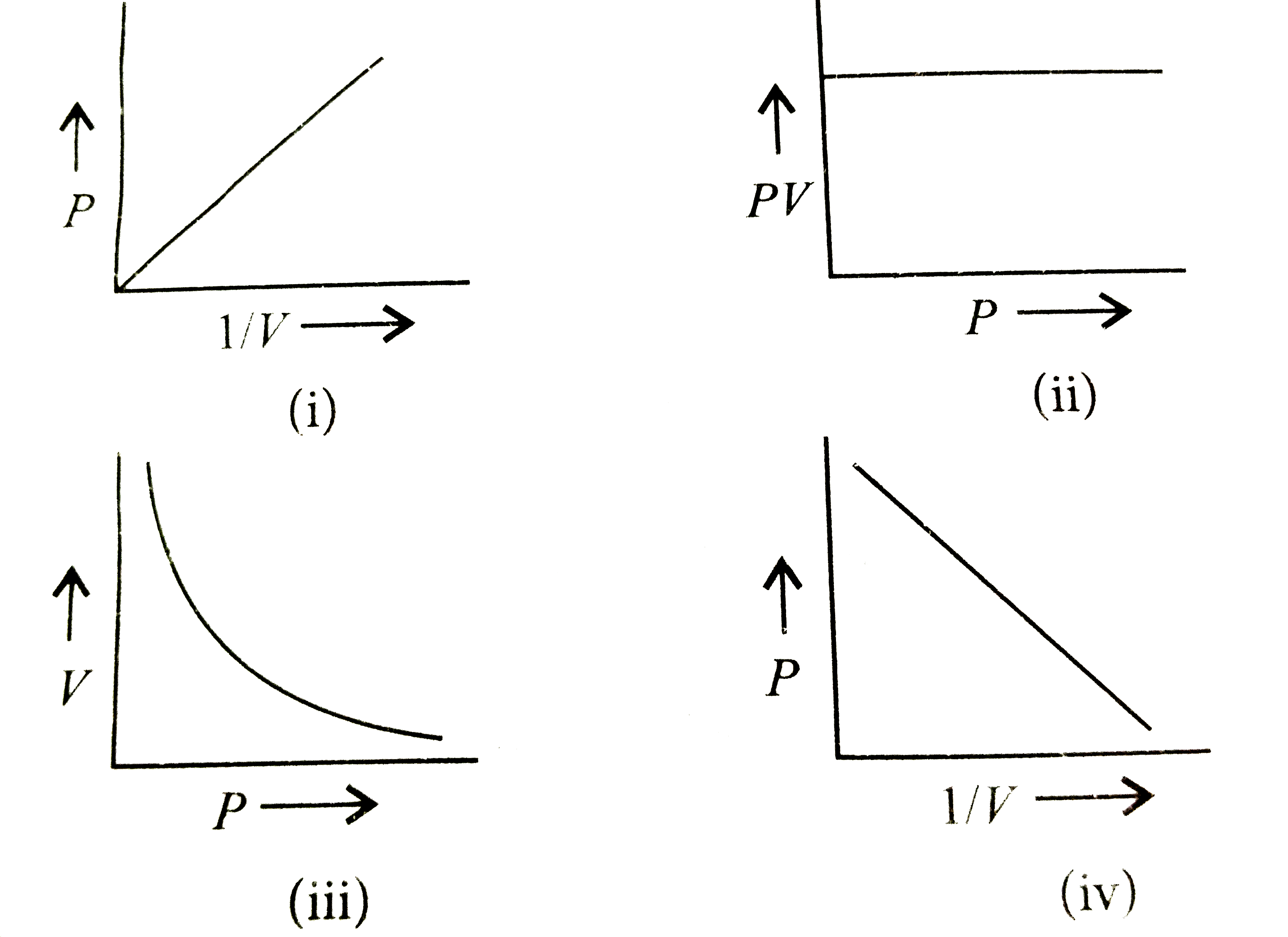
Which of the following graphs represents Boyle's law correctly? (i)
Charles's law implies that the volume of a gas is directly proportional to its absolute temperature. 5.3: The Simple Gas Laws- Boyle's Law, Charles's Law and Avogadro's Law is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The volume of a gas is inversely proportional to its pressure and.

AP Chemistry Boyle’s Law Soxteacher
In 1662, Boyle published version two of The Spring and Weight of the Air. This is were he described the inverse relationship between pressure and volume, now known as Boyle's Law. Boyle's Law states that pressure and volume are inversely proportional to each other. As the pressure increases the volume decreases, and as the pressure.
Boyle’s Law Chemistry Skills
Boyle's law, also referred to as the Boyle-Mariotte law, or, Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas.Boyle's law has been stated as: The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain.

PPT Graphing Boyle’s Law PowerPoint Presentation, free download ID
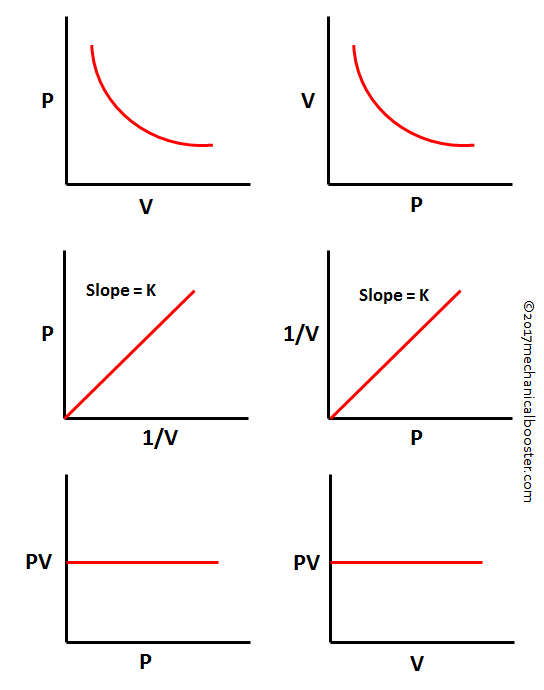
Boyle's Law Graph. There are two conventional graphs to represent Boyle's law. Pressure v/s volume. Pressure v/s volume⁻¹; Graph 1 - Pressure v/s Volume: Represents graph of question pV=K. The value of K is different for each mass of gas, it varies only with respect to temperature.
Boyle' Law
Explore Robert Boyle's 17th-century experiments with gases that led to Boyle's Law. Discover how Boyle's Law, demonstrating the inverse relationship between gas pressure and volume, laid the groundwork for the ideal gas equation. This historical journey illuminates key principles in gas behavior. Created by Ryan Scott Patton.

What are boyles law? Definition, Types and Importance physics AESL
We can use Boyle's law to predict what will happen to the volume of a sample of gas as we change the pressure. Because PV is a constant for any given sample of gas (at constant T), we can imagine two states; an initial state with a certain pressure and volume (P 1 V 1), and a final state with different values for pressure and volume (P 2 V 2).Because PV is always a constant, we can equate.
Boyle's Law Statement, Formula Example and Graph Mechanical Booster
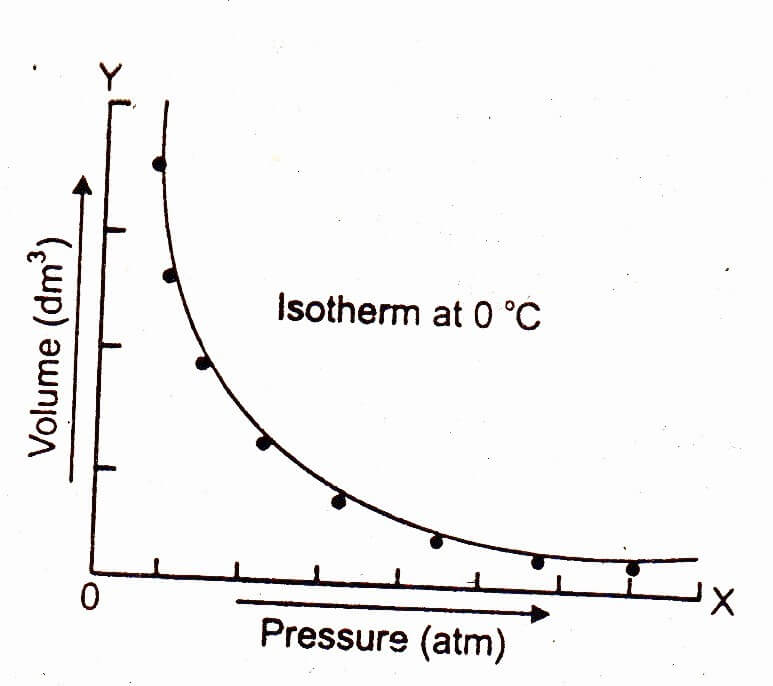
The graph of Boyle's law is known as a pressure-volume graph or PV curve. The pressure and volume graph at constant temperature is known as an isotherm. As the Pressure increases with decreasing in volume, and vice versa. Other parameters such as temperature and amount of gas are constant in the graph. The equation of the curve is PV = K.
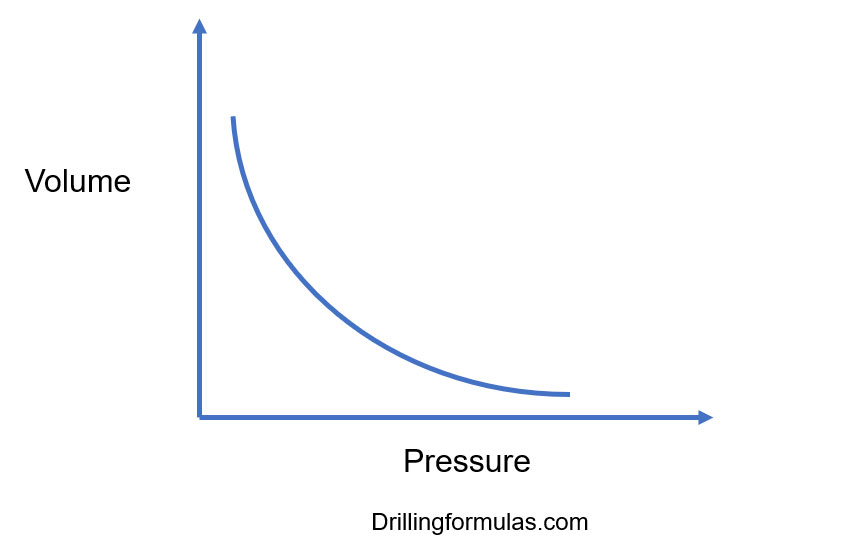
Boyle’s Gas Law and Its Application in Drilling
WARNING! Long answer! Boyle's Law examines the relationship between the volume of a gas and its pressure. > So, you would do an experiment in which you measure the volume of a gas at various pressures. A common lab experiment uses a pop can with various amounts of water to compress the air in a syringe. (Adapted from dwb5.unl.edu) Let's assume you get the following data. ul(bb("Mass/g.
:max_bytes(150000):strip_icc()/boylesdatagraphed-56a129b33df78cf77267fe5d.jpg)
Boyle's Law Worked Sample Chemistry Problem
Explore math with our beautiful, free online graphing calculator. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more.
.PNG)
Gas Laws Presentation Chemistry
Bookshelf ID: NBK538183 PMID: 30844210. Boyle's law is a gas law that describes the relationship between the pressure and volume of gas for a mass and temperature. This law is the mechanism by which the human respiratory system functions. Boyle's law is equivalent to PV = K (P is pressure, V is volume, K is a constant), or one may state.
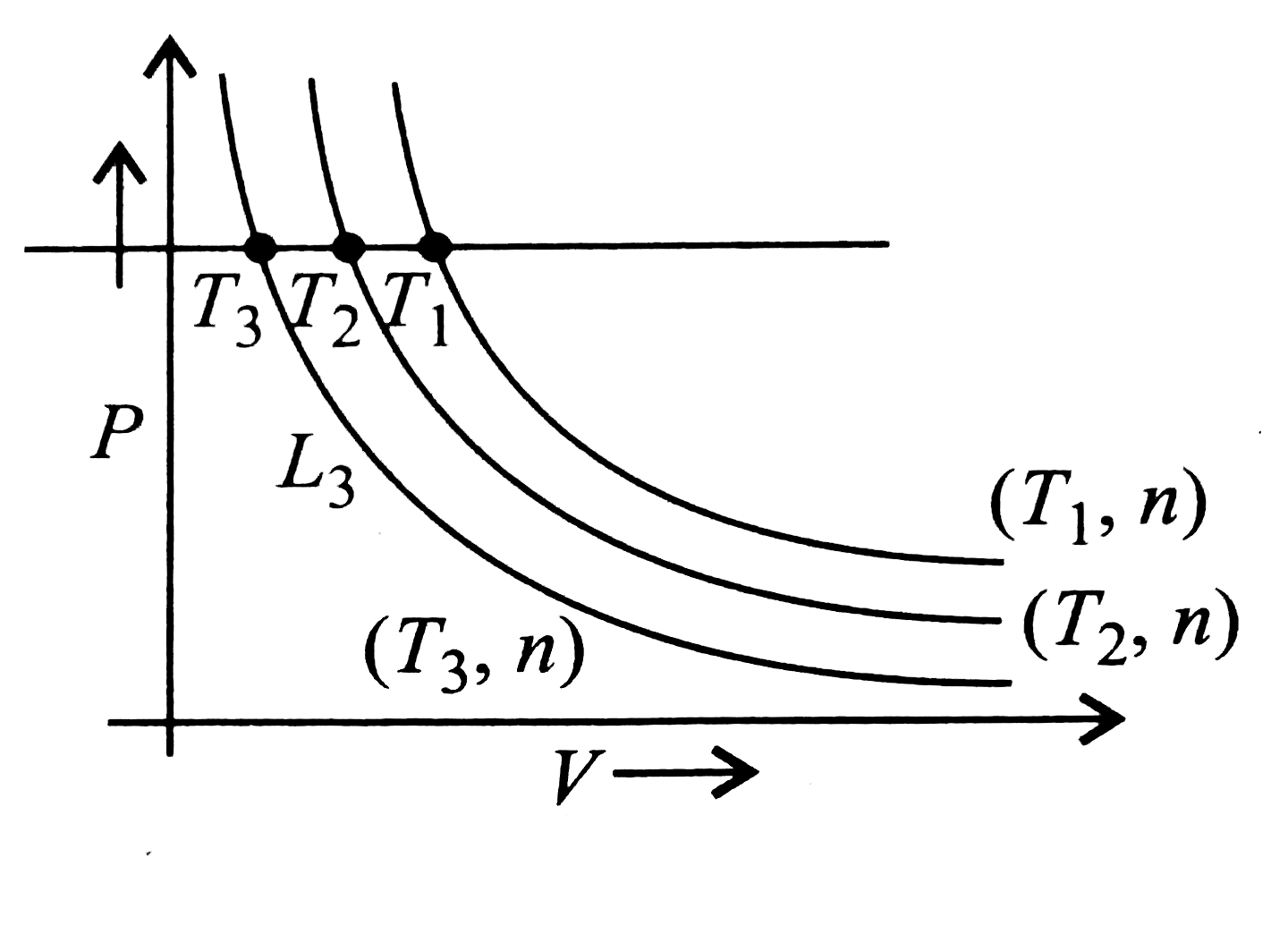
Boyle's Law for an ideal gas can be plotted as shown (rarr) (n moles,
Figure 11.4.1: Boyle's Law. A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right. Boyle's Law is an example of a second type of.